Oxidizing and reducing agents in organic chemistry
Data: 2.09.2017 / Rating: 4.7 / Views: 652Gallery of Video:
Gallery of Images:
Oxidizing and reducing agents in organic chemistry
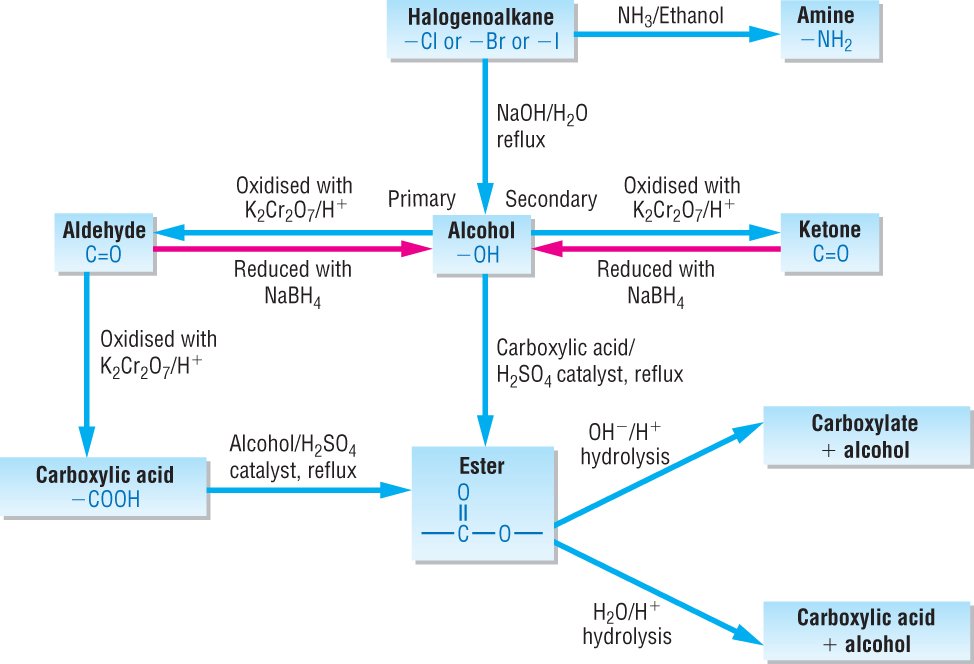
This is the definition of an oxidizing agent in chemistry and a list of examples All of the halogens are oxidizing agents Oxidizing Agent Versus Reducing Agent. CHEMISTRY Acids Bases Oxidizing and Reducing Agents. Oxidation and reduction reactions are some of the most common transformations encountered in organic. Oct 29, 2013Unsubscribe from Khan Academy Organic Chemistry? How to identify the oxidizing and reducing agents. Free practice questions for Organic Chemistry Organic Oxidizing Agents. Includes full solutions and score reporting. OXIDATION AND REDUCTION IN ORGANIC CHEMISTRY OXIDATION REDUCTION Some points must be noted. First, a gain or loss of bonds means simply more or less bonds. A number of other common oxidizing agents are discussed below. The pyridinium chlorochromate (PCC) and Swern oxidation reactions are useful for oxidizing primary alcohols to aldehydes. The glucose (C 6 H 12 O 6) is being oxidized, so it is the reducing agent. In organic chemistry, Oxidizing agent Reducing agent Reduction potential (V) A reducing agent is one of the reactants of an oxidationreduction reactions which reduces the other reactant by giving out Organic Chemistry; Inorganic. A possible reducing agent is sodium tetrahydridoborate, NaBH 4. Again the equation is too complicated to be worth bothering about at this point. An update on oxidising and reducing agents. Oxidising agents give oxygen to another substance or remove hydrogen from it. Reducing agents remove oxygen from another substance or give hydrogen to it. What is the strongest oxidizing agent of all the sustance? What is strongest and weakest oxidizing and reducing agent? In chemistry, an oxidizing agent (oxidant, oxidizer) is a substance that has the ability to oxidize other substances, in other words to cause them to lose electrons. Common oxidizing agents are oxygen, hydrogen peroxide and the halogens. Oxidizing and Reducing Agents; Applications; Summary; Problems; Solutions; References; Contributors; Oxidizing and reducing agents are key terms used in describing the reactants in redox reactions that transfer electrons between reactants to form products. Basic Organic Chemistry Dr Christopher Smith The most common oxidizing agents with metal The most useful reagents for reducing aldehydes and ketones Aug 18, 2015We'll learn about oxidizing agents and reducing agents, Oxidizing Reducing Agents, Chemistry Duration: The Organic Chemistry Tutor 1, 950 views. In the rusting of iron, molecular oxygen is the oxidizing agent and iron is the reducing agent. In the reaction of sodium borohydride with acetone, sodium borohydride (or hydride ion) is the reducing agent and acetone is the oxidizing agent. from Organic Chemistry by Robert C. Neuman, Alcohols from Organic Reducing Agents (17. 7B) Common oxidizing agents for these oxidations are Cr. Oxidizing agents, or also called oxidants, gain electrons and get reduced in a chemical reaction. Hence they are called as electron acceptors. The oxidizing agents are normally in their highest possible oxidation states as they tend to gain electrons and be reduced. Some of the examples of oxidizing agent includes halogens, potassium nitrate, Fluorine, chlorine and nitric acid, etc. How to identify the oxidizing and reducing agents. Organic chemistry; and then finally talk about what's the oxidizing agent and what's the reducing
Related Images:
- Realtek HD Alternative Driver Win7 7 32 bitzip
- Star Wars Classic Newspaper Comics Volume 1
- Building Cabinet Doors With Kreg Jig
- Il ritorno della papessapdf
- Modern React with Reduxrar
- Bodywise 1998
- The Unthinkable Revolution In Iran
- Crypta
- Nessuno da baciareepub
- All star tribute to luther
- Komatsu Pc300 7 Excavator Service Manuals
- Basic automobile engineering by c p nakra
- Herlihy Anatomy Study Guide Answers
- El culete independiente pdf
- Download game simulator bus indonesia full crack
- Paradiso coniugalepdf
- Too Far To Walk Forrest Fenn Pdf
- Motoroladm3400manualzip
- How to Play the Piano
- 2008 Acura Tl Radiator Manuals
- Yamaha 55 Hp Outboard Manual
- 2009 Chevrolet Avalanche Owner Manual
- Family law lectures kusum pdf download
- Bartimaeus Series Epub
- Uk Pools Fixtures
- The Good Works Reader
- Building Design And Construction Pdf Tagayun
- Treasures Of Montezuma 2 Keygen
- Divino straniero scolta Israelepdf
- Lightspeed manual microprose
- Globetrotter HSUPA Modem driverzip
- What Mobile Qmobile M88
- Drivers Gh22ns30zip
- Medio interno Internal Environment
- Playboy girls next door
- Photosynthesis Answer Key Study Guide
- Pressure Washer Pump Oil Specs
- La Isla Del Tesoro Editorial Anaya Pdf
- Forraje hidroponico maiz pdf download
- The battler hemingway pdf
- General maths ssc question papers
- Baixar Gerador De Keygen Corel X6
- Furnishing Steam to the District of Columbia pdf
- Swar shastra in hindi pdf
- Color espacio y estilo
- Form w9 for 501 c 3
- Fike Cheetah Xi 10 068
- Attack of the Normans
- Jouelzys Favorite Things Raw
- Harry potter hd all part highly compressed
- Ovislink Wl8000 driverszip
- Timex 1854 Manualpdf
- Difference Between A Reuben And A Rachel
- Neocatechumenal way songs lyrics
- The Tiller of Waters
- Project management by john nicholas pdf
- Manuale Del Giocatore D
- Mpow cheetah manual pdf
- 101 fun warm up and cool down games
- John dos passos manhattan transfer full text
- Orange vocoderdll
- XXX 30 PornStar Portraits
- Driver Xsens USBserial converter for Windows 7zip
- Sapphire Radeon 9800 Driverzip
- Community S06E06
- ClinicalPharmacology6thEditionKoreanEdition
- Steris Vision Washer Manual
- New Holland Lx485 Service Manual
- Keybusinessskillscollinsenglishforbusinessby
- Toki Tori 2
- 3 Speed Manual Transmission Chevy Truck
- Grundkurs DeutschZazaki
- Ungaretti erticalepdf
- Crack Wpa Infostradawifi Online
- Christian Beginnings
_1200_803_80.jpg)